A leading UK supplement manufacturer asked RSSL to validate the individual quality control testing methods of its entire portfolio of 50 supplements.
This complex process was vital to secure regulatory approval for the company’s move to a new manufacturing site but needed to be carried out across its extensive range within a short timeframe and limited budget - which meant single method development and validation was not a viable option.
Given the commercial constraints of the project, RSSL took a forward-thinking approach. Working closely with the client, we developed a risk-based model that significantly reduced the number of method validations required but still delivered robust scientific accuracy aligned with regulatory requirements.
Our client first put together an overview of their entire product range, which included every product along with its corresponding ingredients by name and quantity. This enabled us to group the supplements according to format type – capsule, tablet or syrup – and then by similar ingredients and quantities. We could then recommend several models that represented the product range and could be used for method validation.
Crucially, this process included a ‘worst case scenario’ for each group, defined as the product with the highest concentration level of active ingredients. Basing our models on a detailed understanding of product formulations and breakdowns was equally important.

If the recipe demanded 60% bulking agent, for example, we replicated the same proportions in the relevant model along with the equivalent percentage of actives. The creation of a placebo sample for each model (containing all the ingredients except the actives) completed our rigorous analytical framework.
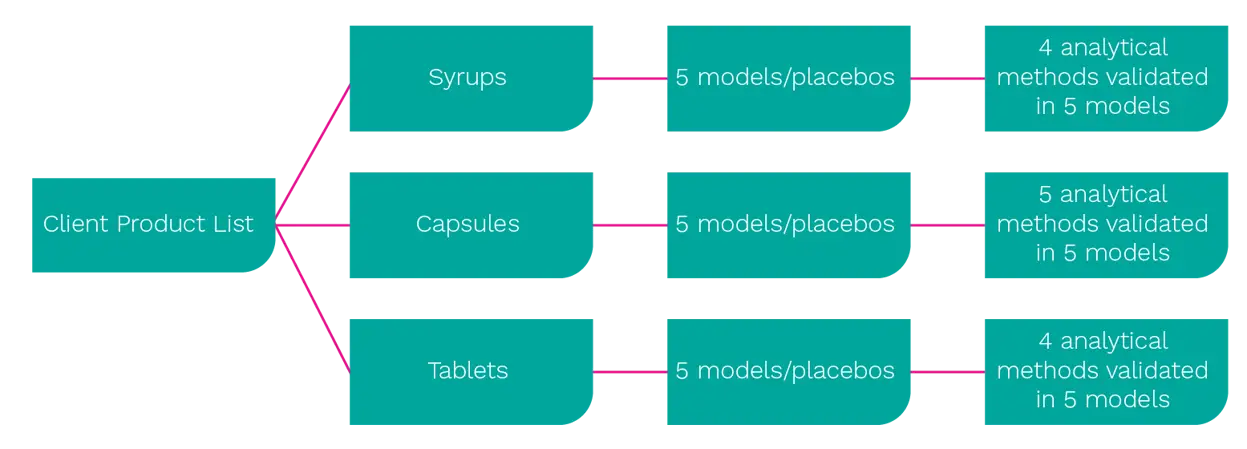
In other words, RSSL’s experienced analytical team translated a highly complex project into four clearly defined stages as summarised below:
Model preparation and selection of representative products: Collating client product data, developing draft models for approval, physical preparation of each model and placebo.
Method feasibility: RSSL’s in-house methods used to assess each active and placebo for sensitivity, specificity, recovery, and linearity. Those developed for vitamins A, E, D, B3, B5 and B6 were deemed suitable. However, vitamins B1, B2, B7 and B12 in capsules required further method development due to low recoveries, while B9 showed a closely eluting peak which caused concern.
Method optimisation/development: Remedial actions taken to address identified issues. This included optimising extraction for vitamins B1, B7 and B12, as well as optimising chromatographic conditions for B9 to remove the impact of the closely eluting interference peak.
Method validation: Performed according to the following parameters: specificity, linearity, accuracy (recovery), method precision (repeatability), limit of quantification and detection (LoQ and LoD).
RSSL’s risk-based approach to this demanding brief cut the total number of models requiring method development and validation by an impressive 75%. We delivered the project on time and on budget, enabling our client to secure regulatory approval to transfer manufacturing to the new site within an exceptionally short timeframe.
Unexpected supplement issues can arise at any point. The range of our analytical expertise means we can head off almost any issue that you will face developing and manufacturing supplements. Find out more about how our talented and knowledgeable team will guide you through your challenges.