A leading pharmaceutical company requested RSSL to assess the compliance of a raw material that they intended to use. The client was in the process of sourcing and assessing different suppliers of Ammonium Sulphate from around the world and as such, sent several different batches to RSSL for full testing to USP requirements.

Not every manufacturer has the resources, expertise, or equipment available to conduct raw materials testing and interpret the results. At RSSL, we have expertise to apply pharmacopoeia monographs, transferring in methods, or developing new methods on client’s behalf. We also provide GMP QC testing services for your APIs, excipients and drug products which is what our client required.
During QC testing, our team within the Wet Chemistry laboratory discovered that some of the batches of Ammonium Sulphate contained high levels of Nitrates and did not meet the acceptance criteria for the Limit of Nitrates to USP pharmacopoeia. Nitrates, which is an impurity from the manufacturing of Ammonium Sulphate, can be reduced to Nitrites by bacteria cultures that naturally occur in the human body.
The high level of Nitrates provided a problem as Nitrites are one of the precursors for Nitrosamines formation which are compounds known for their toxicity.
As a result of receiving the Out of specification (OOS) results, the client requested RSSL to conduct process mapping of the procedure from start to finish. An extensive investigation was carried out to assess the validity of results obtained during QC testing for the Limit of Nitrates. This test consists of an anion exchange reaction of any nitrates present with brucine sulphate. The result is the formation of a yellow complex, brucine nitrate, which can be quantified by UV-Vis spectroscopy at 410 nm. Several parameters of the test were investigated, and results interpreted by the highly experienced Wet Chemistry technical team who communicated data to the client and worked closely with them to decide on the next steps of the investigation.
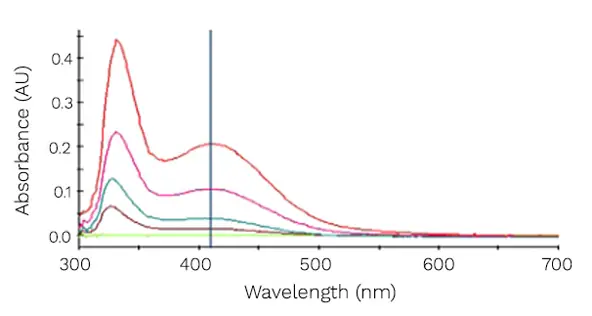
Spectra of nitrate standards by brucine method

PerkinElmer Lamda 650 UV/Vis spectrophotometer
The conclusions of the thorough investigation carried out by the technical experts, validated the original results obtained for two batches of Ammonium Sulphate. These batches were found to contain high levels of Nitrates and therefore did not conform to the pharmacopeial requirements. Our high quality and in-depth investigation helped our client assess and decide which vendors could supply them with raw materials so ensure the quality standards needed for drug manufacturing were met.