Auto-injectors are medical devices designed to deliver a dose of drug through a subcutaneous or intramuscular route. They contain a pre-filled syringe (or cartridge) which is driven by a spring system. The major benefits of these devices are easy self administration, improved patient compliance, dosage accuracy and reduced anxiety.
The global demand for auto-injectors has surged, driven by rising cases of anaphylaxis and food allergies. In such emergencies, immediate administration can be lifesaving, making the reliability of these devices paramount.
Utilising RSSL’s expertise and extensive experience, a comprehensive feasibility study was carried out. The investigation revealed that the spring’s recoil post actuation was interfering with the accuracy of the force measurements – a critical insight into the device’s failure mode.
The activation force is the force required to actuate a self-administered medical device, which is crucial for the device’s proper functionality. If the activation force is outside the specified range, the device may fail to
activate or deliver an inaccurate dosage, potentially harming the patient.
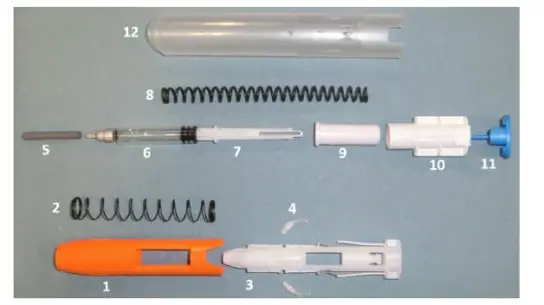
Figure 1: Components of an auto-injector potentially harming the patient.®
Following the results of the feasibility study, RSSL identified a GMP-compliant texture analyser as the ideal tool for this project. A bespoke testing method was developed to ensure accurate and repeatable measurement of actuation force. One of the initial challenges we faced was securing the device in the correct orientation for testing on this instrument. Auto-injectors are complex devices with many internal components as seen in Figure 1, which added to the difficulty.
To address this, the auto-injectors were secured in a bespoke rig designed and created at RSSL. Activation force was applied using a plate surface probe, and a series of tests were conducted to generate force displacement profiles offering a precise measurement of actuation force (Figure 2).
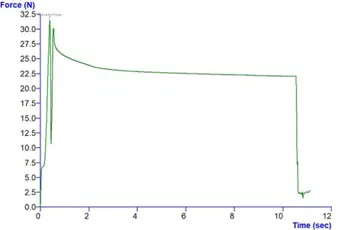
Figure 2. Force displacement profile.
To simulate real-world usage and potential mishandling, additional evaluations were carried out using RSSL’s Zwick testing system. This included dynamic impact testing (e.g. accidental drops) and static load assessments (e.g. compression/ accidental crushing).
While not required for this particular project, injection depth analysis using ballistic gelatine injections of dyed solutions and image analysis tools such as ImageJ or Python can also be conducted where necessary.
RSSL’s experienced team identified the root cause of the performance issue and developed a validated method to measure the actuation force of an auto-injector. This enabled the client to assess the scope of the problem and make informed adjustments to their manufacturing process.