Nitrites and nitrates are commonly found in pharmaceutical products as preservatives or stabilizers. However, the presence of these compounds can lead to the formation of potentially carcinogenic nitrosamines. Nitrosamines are formed when nitrites or nitrates react with amines, which can be present in drug substances, excipients, or contaminants.
The control of nitrosamine formation is essential to ensure the safety and efficacy of pharmaceutical products. Nitrosamines have been identified as significant impurities in some drugs, including ranitidine, which resulted in product recalls and regulatory actions worldwide.
Nitrite and nitrate analysis is important in controlling nitrosamine formation as it helps to monitor the levels of these compounds in pharmaceutical products. By measuring the concentration of nitrites and nitrates in drug substances and finished products, manufacturers can identify potential sources of nitrosamine formation and take appropriate measures to prevent it
High pressure ion chromatography with conductivity detection was identified as a solution, as it is already established within the food and pharmaceutical industries, with the ability to quantify multiple anions from one analytical run. Recent advances in column technology, coupled with recent investment in new instrumentation at RSSL, allowed us to develop a method with a far improved sensitivity than existing anion methodology. We now have the capability to detect a limit down to 1 ppb.
Samples were either diluted in or extracted with Milli-Q water. The range of sample materials which required testing meant method repeatability and recovery had to be assessed for each sample type. The method is considered robust, and if required, can be easily validated for routine quality control release testing.

Figure 1: ICS-6000 instrument, Thermo Fisher Scientific
When diluting the samples, materials freely soluble in water required only dilution in Milli-Q water, however some water-soluble polymeric excipients (e.g. povidone) were shown to stick to the analytical column, which did effect the overall sensitivity of the method.
Fat-soluble excipients (e.g. magnesium stearate) were assessed using both a direct aqueous extraction, but also using an organic/aqueous back extraction. Results were shown to be comparable, but it was noted that the process of back-extraction increases the chances of low level contamination from the environment. As such, a process blank was undertaken alongside all sample extractions.
Materials submitted by the client containing significant levels of chloride impacted the accuracy and sensitivity of the method due to chloride eluting immediately prior to nitrite. Unfortunately, the gradient cannot be further optimised, however, to remedy this, a switch from conductivity to UV/vis detection allowed for the interference from the chloride peak to be removed. RSSL are further investing in a new instrument in 2023 which will allow for this approach to become routine for samples containing chloride.
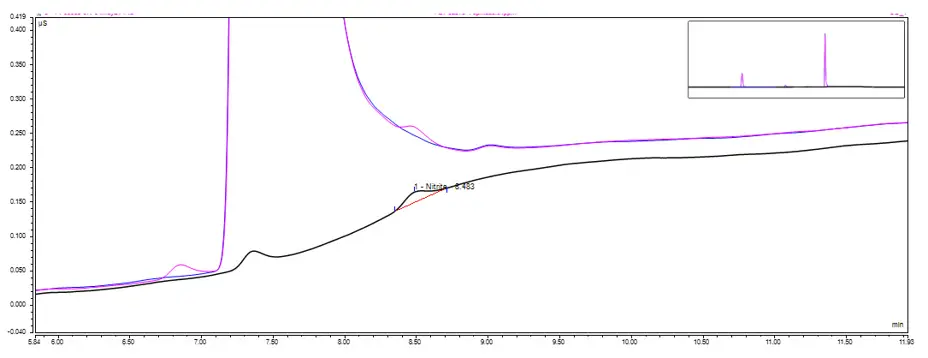
Figure 2: Overlaid chromatogram of nitrite standard (black), spiked (pink) and unspiked (blue) sample containing high level of chloride.
RSSL were able to develop a highly sensitive method of nitrite/nitrate analysis which was applied to a range of pharmaceutical API and excipient materials using high pressure ion chromatography with conductivity detection. The method has since shown to be suitable for a range of materials and going forward, can be validated as per individual client requirements.
At RSSL, we have the knowledge and capabilities to support analytical method development and validation for a wide range of formulation types throughout the drug development lifecycle, including nitrosamines. Our cross-laboratory, multi-disciplinary approach make us an ideal analytical support partner.