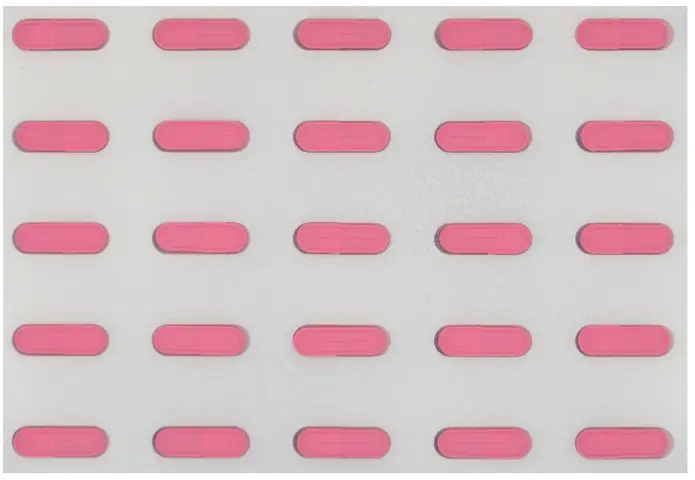
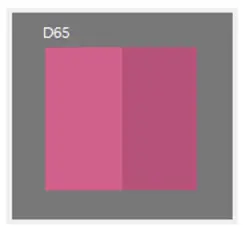
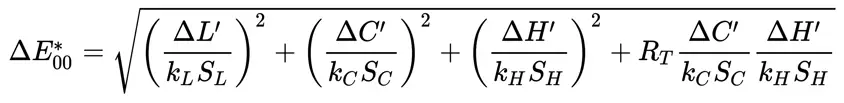A client wanted to ensure colour consistency in pharmaceutical capsules, following regulatory required modifications to the capsule formulation.
Any variation in capsule and tablet colour could raise concerns among healthcare professionals and patients, as colour shifts may indicate broader changes in the product. Therefore, it was critical to assess and maintain consistent colour stability across batches, ensuring that any potential changes remained within acceptable limits to uphold product integrity and trust.
To evaluate potential colour discrepancies, a series of comprehensive tests can be conducted. These tests ensure that any variations in colour are detected and assessed, whether they occur during production or as a result of exposure to external conditions once the product leaves the factory.