The oldest known agent for the inactivation of microorganisms is heat. This important discovery is traceable to when people first started to boil food to avoid food poisoning. The mechanism by which populations of microorganisms are inactivated at high temperatures (in the presence of steam (moisture) and the absence of air), is one where the energy input from the steam inactivates microorganisms by the denaturation and coagulation of their intracellular protein.
The thermal inactivation of microorganisms is one of the most widespread and commonly used means of sterilisation. But what exactly is happening?

Thermal inactivation is associated with the irreversible denaturation of membranes, ribosomes and nucleic acids. However, the precise patterns of macromolecular changes that induce the cell death of microorganisms during heat treatment are still not fully understood. Therefore, our knowledge of precisely what is happening in sterilisation processes comes from laboratory experiments using microorganisms in pure culture. Most microorganisms are destroyed by heat in a process called ‘coagulation’ and moist heat causes irreversible damage to proteins within the cell structure of the microorganism.
In a typical experiment, a pure culture of microorganisms is homogeneously dispersed in a non-nutrient non-inimical fluid, or on inert solid carriers such as paper or aluminium strips.
The numbers of viable microorganisms are counted (usually by plate count methods) at the outset of the experiment. The culture(s) are then exposed to the sterilisation treatment and aliquots or replicates are removed at time intervals. Numbers of viable microorganisms are counted at each time interval.
This type of approach applies to all sterilisation (as well as disinfection) experiments. With thermal processes the cultures would normally be held at constant temperature and samples tested at time intervals. With chemical sterilants (such as ethylene oxide) or disinfectants, the cultures would be held in contact with a particular concentration of the active agent, with samples withdrawn for counting at time intervals. With ionising radiation, the cultures would be placed in a radiation field of known dose rate and samples tested at time intervals that correspond to absorbed doses.
Results are plotted on a graph with the logarithm of the number of viable microorganisms on the ordinate and the time of exposure (or absorbed dose in the case of ionising radiation) on the abscissa. This is called a semi-logarithmic plot. The general form of inactivation of microbial populations when plotted semi-logarithmically is linear. The terms used to describe this are logarithmic or exponential inactivation or death.
Figure 1 is a generalised semi-logarithmic inactivation plot illustrating exponential inactivation (or logarithmic death). The key characteristic of exponential death curves is the slope of the line.
The term D-value is used as an expression of the slope of the inactivation curve. The D-value (or D10 value) is the time in minutes required to achieve one logarithm or 90% reduction of the population of the microorganisms used as a biological indicator under specified lethal conditions.
In thermal studies the D-value should always be qualified by the temperature to which it applies, hence D121-values relate to D-values determined at 121oC, D115-values relate to D-values determined at 115oC and so on.
In radiation inactivation the D-value (commonly called the D10-value for this application) is expressed in units of absorbed dose instead of time, otherwise it is exactly the same as other D-values.
The reason for this regularity of inactivation is attributed to the loss of viability at the biochemical level caused by a reaction/reaction of the First Order (the kinetics of microbial inactivation are said to be pseudo-First Order kinetics).
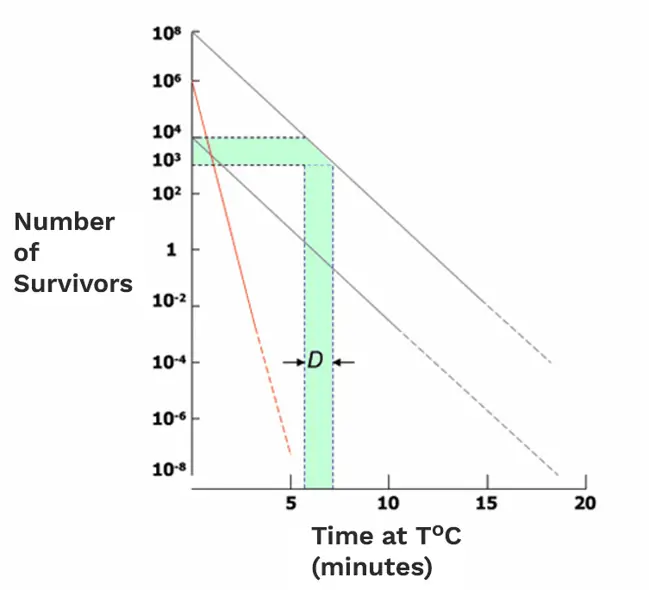
Figure 1: Microbial inactivation graph
What is known from such studies is that bacteria subjected to heat are killed at a rate that is proportional to the number of organisms present. The process is dependent both on the temperature of exposure and the time required (at this temperature) to accomplish the desired rate of destruction. Thermal calculations thus involve the need for knowledge of the concentration of microorganisms to be destroyed.
D-values differ amongst various types of microorganisms. In Figure 1, there are three survivor curves drawn. Two have the same slope but start from different initial populations – this represents how, for one particular pure culture of a microorganism treated under particular specified conditions, the D-value will always (within limits) be the same. The other, much steeper, survivor curve represents a different microorganism, more sensitive to the sterilisation treatment and hence with a smaller D-value.
Irrespective of the method of sterilisation, bacterial endospores are more resistant to inactivation than Gram-positive bacteria and Gram-positive bacteria are more resistant than Gram-negative bacteria. Moreover, resistance is to a lesser extent a function of the environment in which the microorganism is present during the inactivation process, e.g., suspended in water, dried on a filter medium, etc. Environmental effects are not predictable - they must be determined empirically. For instance, there is data (often difficult and confusing to interpret due to heat transfer factors playing a part) to suggest that thermal D-values for bacterial endospores may be up to two times greater when dried on rubber than when suspended in water.
Predicting Sterility Assurance Levels (SALs) for items with different types of contaminants, and even with several types of contaminants at the same time, is not simple. The SAL is a term used to describe the probability of a single unit being non-sterile after it has been subjected to the sterilisation process.
For instance, a population of items each initially contaminated by 103 endospores would require a 9 log inactivation to provide a 10-6 SAL. If the spores had D121-values of 0.5 minutes, this would require a sterilisation treatment of 4.5 (i.e., 9 x 0.5) minutes at 121°C. However, were the items to be contaminated with 102 microorganisms of a Gram-negative species with a D121-value of 0.05 minutes, the same 4.5-minute treatment at 121°C would provide a SAL of 10-88 ([4.5/0.05] -2).
The most widely used sterilisation method in the pharmaceutical industry is steam sterilisation in autoclaves (moist heat in the form of saturated steam under pressure). This is primarily applicable to the terminal sterilisation of products, stainless steel items and equipment not intended for single use. Sterilisation occurs as the latent heat of condensation is transferred to the load causing it to heat rapidly. Steam sterilisation is nontoxic, inexpensive, rapidly microbicidal, sporicidal and is efficient at heating and penetrating fabrics. The penetrating ability of the heat is the reason why steam sterilisation is so widely used as a terminal process for drug products in glass ampoules, vials, syringes and plastic containers.
It is also used for sterilising closures, filters, manufacturing equipment, cleaning equipment, and product holding vessels. Whilst most medical and surgical devices used in healthcare facilities are made of materials that are heat stable and can therefore undergo heat processing, there has been a move towards low-temperature sterilisation methods such as ethylene oxide and radiation as the biopharmaceutical industry embraces single-use disposable items. However, for sterile medicinal products, steam sterilisation remains the most widely used method for products that can be terminally sterilised.
Steam has some very useful properties:
• Long history of use as a sterilant
• It contains lots of heat energy
• Effective against microorganisms
• Contains moisture – shorter hold times
• Good penetration into load items (porous loads)
• Good heat transfer conduction through containers (fluids)
• Non-toxic
• Good process uniformity – easy to control
Steam inactivates microorganisms by denaturation and coagulation of their intracellular proteins and moist heat causes irreversible damage to macromolecules (primarily to cellular structural proteins). It is thought the destruction of cells by lysis may also play a role. Sufficient temperature and time applied to an object cannot only destroy microorganisms in the vegetative state - the optimal combinations can also inactivate microbial endospores.
How steam sterilises
The temperature at which denaturation occurs varies inversely with the amount of water present. It has been found that the presence of moisture significantly affects the coagulation temperature of proteins and the temperature at which microorganisms are destroyed. Therefore, sterilisation in saturated steam requires a precise control of time, temperature and pressure. Devices are required for this specialised sterilisation – autoclaves.
Given sufficient heat applied to an article for a prolonged period of time, dry heat can also effectively destroy a given microbial population. The mechanism of destruction is similar to that applied through steam sterilisation. Dry heat coagulates the proteins in any organism, causing oxidative free radical damage and the drying of cells. Depending upon the time-temperature combination, it can even incinerate the microbial cell. For dry heat to be effective, the items to be sterilised should be free from soiling substances and be dry (since water droplets can interfere with the process, particularly in relation to short sterilisation run times).
The typical response of microbial populations to dry heat is exponential. The slow reaction rates seen in dry heat sterilisation indicate that the mechanisms of microbial inactivation are most likely the results of intracellular oxidative reactions. Because the biochemistry of inactivation differs, microorganisms which have resistance to thermal sterilisation by saturated steam are not necessarily also resistant to dry heat. Spores of Geobacillus stearothermophilus, used to evaluate steam sterilisation, are not as resistant to dry heat as spores of Bacillus atrophaeus. This intrinsic difference affects the type of biological indicator used for the validation of a heat sterilisation device.
The first requirement in developing specifications applicable to a particular dry heat process is to decide whether its purpose is to sterilise only, or to sterilise and depyrogenate (as demonstrated by the inactivation of bacterial endotoxin). With dry heat, it is not possible to depyrogenate without also sterilising.
If sterilisation is the sole intention, the purpose of dry heat process development is to determine time and temperature combinations that will achieve a 10-6 SAL standard or better, or to develop an ‘overkill’ cycle that will deliver twelve log10 reductions relative to challenge endospores.
If depyrogenation as well as sterilisation is required, the purpose of dry heat process development is, for pharmaceutical product-contact components, to determine time and temperature combinations that will affect a standard three Log10 reduction of bacterial endotoxin (based on pharmacopeial requirements).