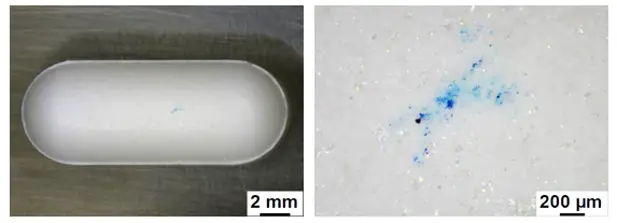
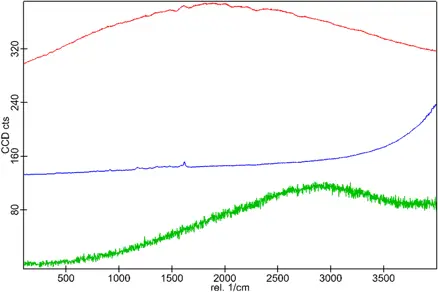
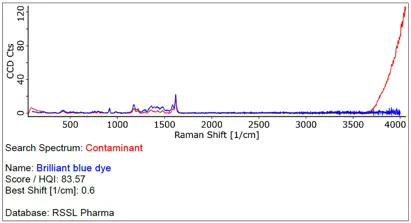
The benefits of Raman Spectroscopy in contaminant identification
In the pharmaceutical industry, contamination can pose significant risks, affecting product safety, efficacy and quality. Rapid and accurate identification of contaminants is crucial for determining whether these issues stem from manufacturing processes and for addressing them before they cause wider problems.
One advanced tool for this purpose is vibrational spectroscopy, a group of analytical techniques that provide molecular-level information on how materials vibrate, leading to their identification. Confocal Raman spectroscopy has emerged as a powerful and precise method for identifying contaminants, especially in cases where high spatial resolution is needed.
The power of Confocal Raman Spectroscopy
Confocal Raman spectroscopy offers a distinct advantage over traditional spectroscopic techniques, such as Fourier Transform Infrared (FT-IR) microspectroscopy and Scanning Electron Microscopy